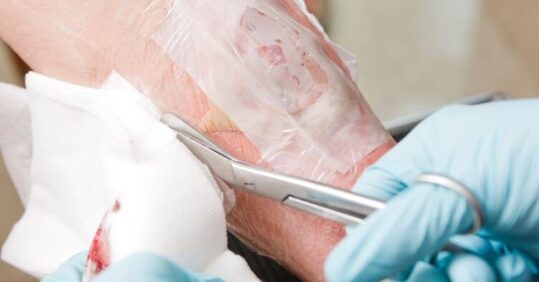
Nurse tissue viability lead Christina Harris explains the role of larval therapy in wound management and its potential uses in the community
Larval (maggot) therapy is increasingly being recognised in the NHS as an important method to support wound healing both in hospital and community settings. However, some research suggests that nurses outside specialist settings need more support and education to gain confidence in using this approach.1
This article provides an overview of the main principles of larval therapy in wound management, its potential applications in community settings and how it is used in practice.
How does larval therapy improve wound healing?
Larval, or maggot, therapy is used for debridement of wounds – debridement being the removal of devitalised (dead) tissue – eg, necrotic slough or haematoma.2 The presence of unhealthy or dead tissue can add to the risk of infection and delay wound healing, and debridement is an important part of wound bed preparation.3,4 The body can do this naturally through autolysis, but this can take time. In patients with co-morbidities such as diabetes or diseases which may affect oxygen perfusion, delayed healing is common and any barriers to healing should be addressed in a timely way.
Larvae of the green bottle fly (Lucilia sericata) are used in larval therapy for wounds. The larvae feed on dead tissue, cellular debris and serous drainage (exudate) present in sloughy wounds. Their characteristic feeding action breaks down necrotic, haematoma or sloughy tissue, which is then consumed and digested. The process involves both physical actions of the moving larvae surface and proteolytic enzymatic digestion. The larvae feed mainly by a process of extracorporeal digestion, secreting enzymes which breakdown devitalised tissue into a semi-liquid form that they then ingest. Crucially, L sericata larvae do not digest healthy human tissue, and this selective process is one of the major advantages of larval therapy.5,6
Other methods of debridement include surgical debridement under local or general anaesthetic, sharp debridement or enzymatic methods. The choice of debridement method depends on a thorough assessment of the patient and wound, and should be tailored to the needs of the individual. Patients who are cared for in the community may have limited options – mechanical and sharp surgical debridement are fast and popular but can be unselective, painful, carry a risk of bleeding, require anaesthesia and must be performed by a skilled practitioner in a safe environment.2,7,8
Larval therapy is a recognised option for rapid debridement of devitalised tissue in patients cared for at home or in a care home, where admission to hospital for sharp or surgical debridement may not be favourable. Larval therapy has also shown to be cost effective when compared to other mainstream debridement interventions including surgical, sharp, mechanical and natural autolytic (aided by dressings) debridement methods.9
When is larval therapy recommended?
Larval therapy can be used safely on many wound types including pressure ulcers, leg ulcers and diabetic foot ulcers.10 The larvae can also be used in traumatic haematomas, but if a patient is on anti-coagulants (blood thinners), it is important to ensure their clotting factors are within acceptable levels or bleeding is a potential complication. This is particularly important in a community setting.
Patient location should not limit access to treatment.11 However, if the larvae are applied to a weight-bearing area, such as the feet or sacrum, that area needs to be completely offloaded which is not always easy in a patient’s home and may even pose a risk of falls. In this case, a full risk assessment should be undertaken in partnership with the patient, their families or those caring for them, and their care may need to be tailored during the period of treatment to obtain the best outcome.
In general, once full patient and wound assessments have been completed and contraindications have been excluded, larval therapy should be very safe under close supervision of the community nursing or the podiatry teams. These teams should be competent and confident in the application of larval therapy and the ongoing care throughout its wear time.
How is it used in practice?
The sterile larvae are applied to wounds in bags made of netting and covered with a non-occlusive dressing so that the larvae do not suffocate. (In the UK there is only one company that manufactures the sterile larvae for wound management, and they now only supply them in the bags known as ‘BioBags’ rather than as loose larvae). The bags can be obtained in various sizes depending on the size of the wound – a handy online calculator can be used to aid selection of the most appropriate size, which depends on aspects such as the depth and surface area of the wound.
Once the decision has been made that larval therapy is the most appropriate treatment, nurses provide the patient with relevant information and ensure they have understood and consented to its use, all fully documented in the patient’s case notes, before proceeding.
In particular, patients should be informed that wound exudate may increase, which may appear ‘pinkish’ in colour, and that there may be an unfamiliar odour during treatment (all of which is normal) – so that they know what to expect.
The patient’s GP will then need to prescribe the larval therapy before it is ordered. It is best to avoid starting any new therapy at the end of a week as fewer services are available over a weekend should there be any problems, so it is sensible to order the treatment in time for delivery on a Monday.
The day of application is considered day 0 and the treatment is usually put in place for 4 days, during which time the patient should be monitored daily for any unwanted effects such as bleeding, pain or discomfort. Larvae should be observed at each dressing change to ensure they are still viable. The progress of debridement should also be noted at this time to ascertain if a further bag needs to be ordered to avoid any lapse in treatment. The outer dressings should be changed, surrounding skin treatment re-applied and damp gauze replaced.
After treatment, the larvae are disposed of in the general waste.
The images below show the progression of a traumatic haematoma treated with larval therapy. Debridement occurred over two applications of a BioBag 2 cm x 10 cm x 10 cm. Within 10 days the wound bed was completely debrided. Note that, given the size and depth of the wound, the observed reduction in devitalised tissue would take weeks or months to achieve if relying on natural autolytic debridement and dressings.

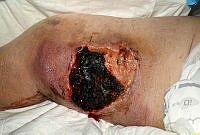
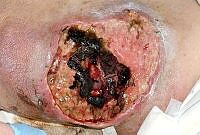
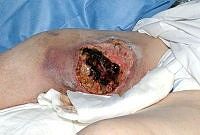
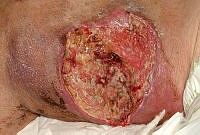
Images: Author’s own, reproduced with permission
How do patients feel about larval therapy?
Patients are generally accepting of larval therapy once all the relevant information is given and understood. Often, when they see how effective the treatment is, they are very positive about their use.
Pain or discomfort is rare, but some patients report they are ‘aware of them being there’. This is usually relieved by taking simple analgesia, such as paracetamol. A positive attitude by the nurse during application is important to prevent any preconceived ideas becoming a barrier to tolerance.12
Nurses considering larval therapy will find the larvae are delivered with full instructions and a patient care plan, but if practical support is needed their local tissue viability team may be able to offer advice.
Christina Harris is Tissue Viability Lead at University Hospital of Wales
References
- Hopkins R, Williams S, Brown A et al. Evaluating nursing opinion and perception of maggot therapy for hard-to-heal wound management. J Wound Care 2022; 31(10):
- Strohal R, Dissemond J, O’Brien et al. (2013) EWMA Document: Debridement: An updated overview and clarification of the principle role of debridement. J Wound Care 2013;22(Suppl 1): S1–52
- NICE. Pressure ulcers: prevention and management. [CG179] 2014
- Fletcher, J. Wound bed preparation and TIME principles. Nurs Standard 2005;20(12): 57-65
- Fleischmann W, Grassberger M, Sherman R (eds) Maggot Therapy: A Handbook of Maggot Assisted Wound Healing. (2004); Stuttgart: G Thieme Verlag
- Gottrup F, Jørgensen B. Maggot debridement: an alternative method for debridement. Eplasty 2011;11:e33
- Wolff H, Hansson C. Larval therapy – an effective method of ulcer debridement. Clin Exp Dermatol 2003;28(20):134-7
- Mudge E, Price P, Walkley N et al. A randomised controlled trial of larval therapy for the debridement of leg ulcers: Results of a multicentrer, randomized, controlled, open, observer blind, parallel group study. Wound Rep Regen 2014;22(1): 43–5
- Bennett H, Sewell B, Anderson P et al. Cost-effectiveness of interventions for chronic wound debridement: an evaluation in search of data. Wounds UK 2013;9(4 Suppl): 3–11
- Chan DC, Fong DH, Leung JY, et al Maggot debridement therapy in chronic wound care. Hong Kong Med J 2007;13(5):382–6
- Atkin L, Acton C, Edmonds M et al. The role of larval debridement therapy in the management of lower limb wounds. Wounds UK 2020
- Kitching M. Patients’ perceptions and experiences of larval therapy. J Wound Care 2013;13(1): 25-9